A Noninvasive Treatment for A Progressive Neurodegenerative Disease
Huntington’s Disease: proof of concept
- Noninvasive IV injection of nanoparticles of the trojan-horse complexed electrostatically to an ASO.
- Very low effective dose (1/100th of the dose used intrathecally by competitors).
- No signs of toxicity during in vivo rodent studies up to 6 months.
- Halts the progression of Huntington's disease in the most severe humanized mouse model (Figure 1)
- Reduces Huntingtin protein expression in different brain regions in mice and monkeys (Figure 2).
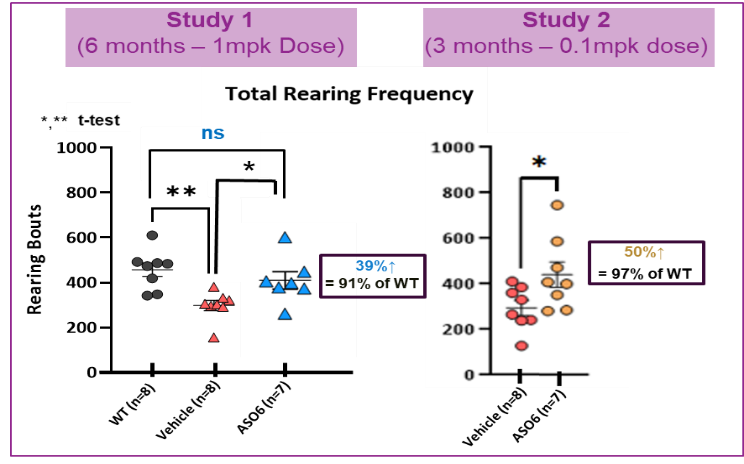
Figure 1. Halts the progression of Huntington's disease. Restores normal rearing activity in zQ175 humanized Huntington’s disease mouse model with 1 mpk (Study 1) or 0.1 mpk (Study 2) doses of Ophidion’s lead Trojan horse-linked Huntingtin ASO. The Trojan horse-ASO was administered IV bimonthly for 6 months in Study 1 and for 3 months in Study 2.
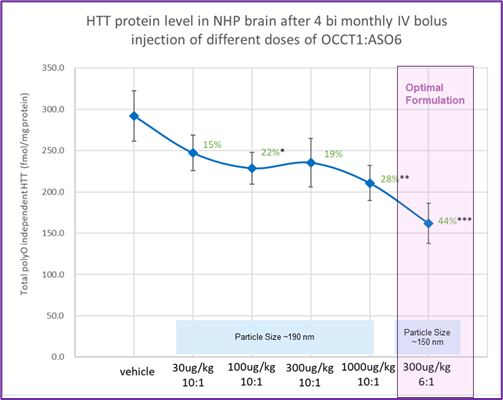
Figure 2. Reduces Huntingtin protein (HTT) expression in different brain regions. Significant reduction of soluble wild-type HTT protein in various brain tissue (hippocampus, striatum, motor cortex, and somatosensory cortex). One-sided T test: *p<0.1, **p<0.05, ***p<0.01Each data point n = 8, error bars shown are standard error
